
Accelerating Decentralized Clinical Trials Adoption
elluminate Academy is a monthly webinar series featuring life sciences experts and eClinical Solutions thought leaders.
Recently, our Chief Marketing Officer, Sheila Rocchio, sat down with Jennifer Price, Executive Director of Data Analytics from THREAD Research, and eClinical’s Dawn Kaminski, Senior Director of Data Strategies, to discuss the need for decentralized clinical trial (DCT) strategies that improve results and get therapies to market faster.
Watch Now
The Pivot to Decentralized Clinical Trials
Decentralized clinical trials include the use of digital tools to capture consent and collect data, remote patient monitoring and diagnostics, and in some cases the move from central lab to local lab locations. This is a sharp contrast to the traditional clinical trial models that requires patients to interact with doctors at in-person site visits. The status quo significantly reduces the number and the range of patients who can participate in clinical research because of issues related to proximity and convenience, as well as patient recruitment strategies that are focused on access to a physical site.
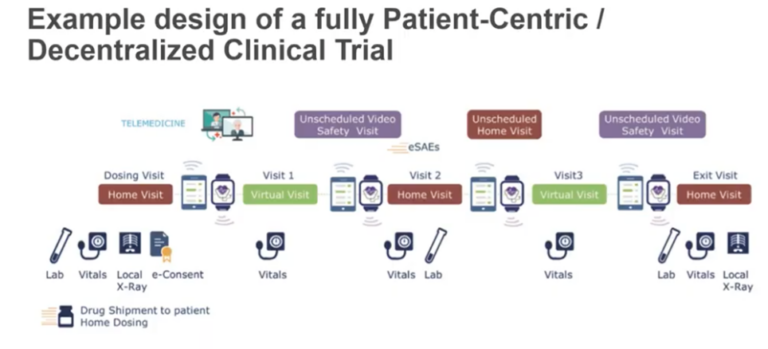
*Source: SCDM Short Brief Decentralized Trials
Throughout the Covid-19 pandemic, it was nearly impossible to conduct in-person site visits due to medical constraints, health and safety concerns. Due to this, cinical trials that relied on traditional models were delayed or canceled. Those that managed to continue did so because they were able to pivot to hybrid decentralized approaches, often by using virtual visits to interact with patients involved with the trials. The results were positive and allowed trials to continue with a more patient-centric approach. The success achieved by rapidly moving to decentralized trial models at scale is fundamentally changing how the drug development industry, technology providers and health regulators are considering the future of clinical trials.
The Future is Now
Jennifer Price (THREAD Research) shared that current trials have the opportunity to pivot to DCT or hybrid approaches by including the following:
- Add eConsent (BYOD and/or provisioned device)
- Add on-demand Telehealth Virtual Visits options
- Add flexible scheduling allowing visits to be conducted via telehealth, home health and/or on-site
- Add source document collection and management to support data capture during Virtual Visits
- Move on-site assessments to technology-enabled activities
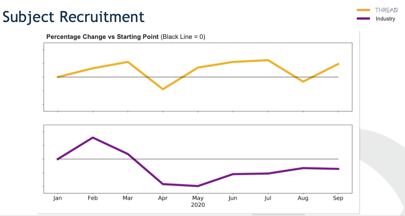
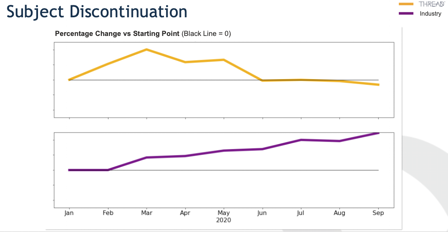
How DCTs Impact Data Review and Management
There are unique challenges that stakeholders face when conducting trials reliant on telemedicine, sensor and digital health data, and local labs, since a far greater percentage of the data is eSource. This paradigm is fueling the changing both the role of data management and the technology infrastructure used to facilitate the data review processes. The SCDM Short Brief on Decentralized Trials states that “in this context [of fully decentralized trials], most of the traditional data cleaning processes are not applicable. Additionally, there are no site monitors to handle data‐related issues upstream on CDM’s behalf. Due to the limitation of addressing data issues after collection in a DCT model, the process heavily relies on technologies collecting error free data with quality checking at the time of data collection. Data processing would be focused on data consolidation from diverse technologies and sources rather than data cleaning. So, clinical data scientists should proactively assess data mastering and data reconciliation keys across modalities.”
The recent pivot to decentralized trial platforms catalyzed by the pandemic has accelerated this transition. Now more than ever, there is a need for a single centralized source of truth for all study data in order to accelerate review and analysis. As Dawn Kaminski described, “Holistic data review across all sources using platforms like elluminate is essential, as are risk-based approaches that help data reviewers rapidly identify trends and outliers.” Analytics can show which data points are most unlike others and require additional inquiry. Platforms like elluminate can help data scientists pinpoint outliers, enabling a more directed review and analysis of data. Additionally, the need for interoperability with numerous data collection systems has never been more critical. Decentralized trial designs may rely on even more disparate data sources to support remote participation. It is imperative that data management technologies advance alongside new clinical trial designs.
Although we are not yet at a point where DCTs are the norm, emerging technologies and new priorities are helping the industry make the pivot. Says Jennifer Price, “I would love in a few years to say decentralized trials are the traditional clinical trial model, because we’re certainly seeing a lot of action in converting some of the traditional trial items to a decentralized model.”
To learn more about decentralized clinical trials, the “Accelerating Decentralized Clinical Trials Adoption with Modern Platforms” webinar is available on-demand.
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.







