
Developing a Data Strategy Roadmap as the Foundation of Your Digital Trials
Clinical trials are taking new forms every day as the industry undergoes a shift brought on by the influx of new technologies, a greater variety of data types, and a higher frequency of decentralized and hybrid trial models. Clinical Data Managers need to develop an agile data management strategy that can work for the current state of clinical trials and be flexible enough to adapt to the digital trials of the future. Identifying and creating a data strategy roadmap in the midst of these growing pains can present a challenge, but there are several steps life sciences organizations can take now to ensure success today to be future ready for the changes that are coming quickly.
Understand and Prepare for Decentralized and Hybrid Clinical Trial Models
The onset of decentralized clinical trials was largely advanced due to the necessity of alternative trial models following the Covid-19 pandemic. There’s a need for a clinical trial solutions that can reach a greater number of patients while ensuring high data quality review and analysis. Improving the overall patient experience is a motivating factor for decentralized trials, as is easing the burden of traveling to an investigator site. While the industry is undoubtedly moving toward remote and hybrid approaches to trials, many organizations lack the infrastructure to accommodate the shift.
Operational leaders are plagued by oversight and monitoring challenges in a decentralized model, while spending more time finding effective ways to streamline and standardize data received from non-traditional sources. eClinical’s CEO and Co-founder, Raj Indupuri believes interoperability will be essential in achieving a successful transition to virtual trials. “Closed systems will not work. At an enterprise level you could have multiple hubs – a hub for clinical data, one for supply chain data, and one for your discovery data. When you factor in decentralized trials, there is so much innovation happening from different types of instruments and devices, apps, telehealth… the key IS interoperability between all of these systems to ensure that trials mature with modern APIs.”
Improving data quality and increasing patient retention and recruitment should ultimately be the goal when designing for decentralized or hybrid trials. The technology shift needed to accommodate these trials is only part of the process – clinical trial managers must also develop a holistic approach, taking into consideration the patient, stakeholder, and vendor experience when moving away from a traditional trial model. During the elluminate Engage 2021 conference, Ian Shafer, Principal at PwC shared that “this isn’t just technology. It’s the design of the program, its utilization of technology to create a different participant experience, and being mindful around an investigator and what that does to their workflow and how you can help them be more efficient.”
So how do you design a data strategy that not only accommodates technology advancements in decentralized trials, but also support the needs of multiple stakeholders? Investing in a clinical data platform that can ingest data from various sources as well as provide customizable views per roles for increased transparency and oversight is a good place to start. A platform like the elluminate Clinical Data Cloud can help simplify the data review process and harmonize the data streaming in from virtual clinical trials.
Reduce Resource Drain on Data Management Teams
While there is not a one-size-fits-all solution for organizations looking to scale their clinical trials, a critical step is identifying the internal resources needed to sustain growth. For many data management teams, the existing technology infrastructure is lagging, putting more strain on manual processes and making data standardization increasingly difficult.
A surprising number of organizations are still relying on SAS and EDC/Excel trackers for data review – 75% in fact. While these existing processes may have been suitable in the past, as the clinical trial ecosystem continues to change, the traditional methods of data review are no longer sustainable. The clinical data process is only growing more complex with the addition of artificial intelligence, machine learning and other new technologies.
If an organization wants to ensure that their clinical data strategy can keep up with the shifting landscape, then consideration must be given to the capabilities of their clinical data managers. Avoid overburdening your most valuable team members with manual labor by providing them with resources like a clinical data platform that can help sift through the data deluge.
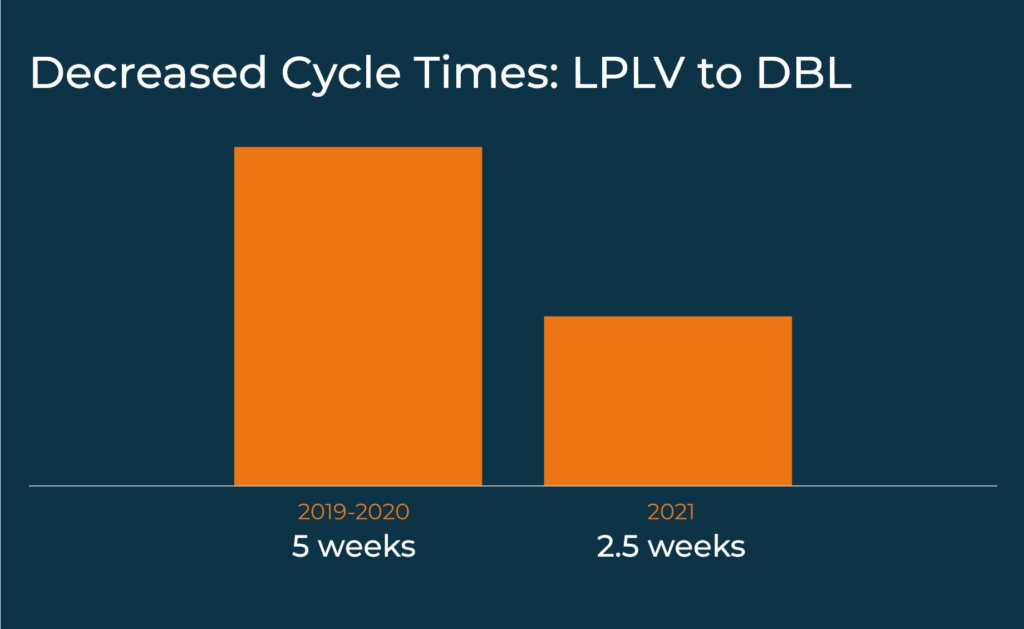
Adapting to a new clinical data software can increase efficiency, decrease data manager burnout, and help achieve higher quality data faster. When eClinical Solutions implemented elluminate within our Data Services team, which provides expert data configuration, management and statistical analysis for clients conducing digital trials, we saw a 50% decrease in cycle times from Last-Patient-Last-Visit to Database Lock in 2021.
Optimize Your Clinical Trial Data with a Centralized Platform
When building upon your existing clinical data strategy, look for tools that can not only aggregate clinical data but help you maximize its value. By leveraging a powerful clinical data platform like elluminate, life sciences organizations can optimize their existing clinical data with 40+ out-of-the-box visualizations, comprehensive mapping and reporting capabilities, enhanced risk-based quality management and more.
Benefits of implementing elluminate to enhance your clinical data strategy include:
- Single access point for all clinical data sources
- Advanced analytics capabilities
- Quicker access to accurate data for end-users
At Bristol Myers Squibb, elluminate was leveraged to streamline clinical data flow as part of a new, future-ready clinical data architecture designed for the increasing volume of external data streams. Using the elluminate Clinical Data Cloud Importer and Mapper module for quicker access to clean data and to create Consolidated Clinical Views (CCVs), BMS was able to streamline data acquisition, mapping and standardization tasks resulting in faster access to data by downstream teams.
David Rucker, Associate Director Business Capabilities, Clinical Data Acquisition & Standards at BMS shared that “the biggest value that we see is being able to pull data into a central location. Right now we have data that comes in from various locations, and we look at elluminate to be the product that’s going to bring all of that data into one place and give us a central repository.”
Build Your Clinical Data Strategy for Future-Readiness
As we look ahead to the future of clinical development, it’s undeniable that the role technology plays in clinical trials will only grow. The core tenets of an adaptive data strategy consist of an interoperable approach to non-traditional trial models, investment in resources to ease the data management burden, and leveraging insights from a robust clinical data platform. The elluminate Clinical Data Cloud can help alleviate the pain points of an outdated clinical data infrastructure by compiling all data sources into a unified source of truth, while creating cross-study analytics reports and enabling deeper insights.
Author

By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.







