
Industry Insights from the Frontline of Digital Transformation
Highlights from the elluminate Engage 2021 Virtual Conference
During the third annual elluminate Engage conference held virtually on November 3-4, over 250 attendees from more than 70 life sciences organizations across 12 countries convened to share insights on new technologies impacting the industry, best practices and use cases for elluminate, accelerating digital transformation, and more. Industry leaders in cross-functional roles shared how they are maximizing the value of clinical trial data with the elluminate clinical data cloud TM .
If you missed the conference, you can still gain access to the on-demand sessions by registering here.
Making Digital Transformation a Reality
Implementing digital transformation initiatives is a growing priority for many data management and clinical operations teams, but the path toward achieving the elusive “transformation” isn’t always clear. Raj Indupuri, CEO and Co-Founder of eClinical Solutions brought together clinical development experts to discuss how they are working toward increased digital fluency at their organizations in the panel, “Digital Transformation and Innovation.” Raj was joined by Greg Moody, Vice President, Research and Development at Biogen, Igor Proscurshim, Vice President, Head of Clinical Development at Ichnos Sciences, and Venu Mallarapu, Senior Director of Life Sciences/Global Head of R&D at Cognizant.
Finding the right balance of what digital transformation means at the individual contributor level and the stakeholder level can be challenging, especially when there is an influx of new technology to integrate into existing processes. Greg Moody shared that digital advancement in the life sciences tends to come in waves, and right now the industry is inundated with new information from non-traditional data sources like sensors and wearables. He shared his insight into how elluminate can help teams invest and maximize the time spent working in the middle of the data pipeline, stating that “a solution like elluminate is the suspension bridge that you’re building for your data engine so that data can come in – you want the flexibility of a good data management pipeline tool to allow you to bring in that diverse set of information, manage it, ride out the bumps, and get it to a usable end point.”
Greg continues to explain that in the hole of digital transformation, it’s easy to forget that people are in the middle of the data management process, between the machines collecting data on the frontend and tools like AI/ML translating the output on the other end. elluminate is where the people working in the middle get to interact with the data, and gain all of the advantages of automation and centralization provided by the platform.

When asked to consider the key outcomes that stakeholders should strive for in digital transformation, Igor Proscurshim emphasized the importance of being able to bring innovation to patients as efficiently and quickly as possible. He explains, “It’s about advancing the science, and getting data in a usable and actionable format as quickly as possible, which is easier said than done. A lot of the data review has a human factor and there’s always room for error in human input, which then has to be corrected and queried. Any tool that enables that to be done in near-real-time like elluminate enables us to have that actionable data sooner.” Faster time-to-market is ultimately the desired outcome, and from a technology standpoint, stakeholders need to ask themselves how to put technical innovations to good use while maintaining quality and compliance, and keep the cost of ownership low. These are the questions Venu Mallarapu believes are at the core of digital innovation and building sustainable practices, which will lead to more therapies reaching more patients faster.
Thoughts on Buying Platforms versus Building In-House
Many life sciences organizations have paused to question the benefits of investing in a built platform that can rapidly provide access to trial data, or if they should invest internal resources toward building their own Electronic Data Capture (EDC) software. There is a strong argument toward buying an out-of-the-box tool, says Greg Moody, who has built a lot of code himself in his career. “I want to be able to focus on small pockets of innovative design that elluminate allows us to plug-in to, while providing guard rails so that we know we have a stable, scalable, reliable and forward-thinking environment that we’re building from.” There is an increased sense of security with clinical data, and more operational leaders are leaning towards a preference for a pre-built tool with interoperability and customization that can securely aggregate numerous data sources. As Igor Proscurshim states, “We should be developing drugs, not developing software. Often whoever proposes the building of an internal software vastly underestimates the complexity of that build.” By investing in a platform like elluminate, internal resources can be freed up to invest more time acting upon data rather than developing the tool to ingest it.
For more insights on the build versus buy conversation, watch these highlights from the panel’s discussion.
Accelerating Technology Adoption
Any time an organization chooses to invest in a new technology there is often varied response from those tasked to use it, from people who can’t wait to get their hands on it to those who are hesitant and unsure of how to adjust existing processes to accommodate it. One of the benefits of a user conference like elluminate Engage is the opportunity to hear how other users of the platform are maximizing and tailoring its benefits for their specific needs.
In the session, “The Innovation Imperative: The Need for Clinical Systems Interoperability and Analytics”, Megan Dunham, Associate Director of Clinical Innovation, and Vijay Koduru, Associate Director of STAT Programming from Jazz Pharmaceuticals shared a case study for how Jazz integrated elluminate into their existing clinical data factory architecture.
Megan shared that like many biopharmaceutical companies, Jazz had existing data collections processes that only marginally changed when collection methods shifted to digital through EDC. In order to advance innovation, Megan’s team “knew with the implementation of a novel platform that we had to challenge the status quo and break clinical trial team members from functional silos. And perhaps, the biggest challenge of all, we had to change user behavior – which means motivating them and making it easy enough to successfully change and form new habits for how to work with data.”
When initiating elluminate adoption for Study Data Tabulation Model (SDTM) mapping and visualizations, Vijay expressed that identifying change agents was one of the post powerful ways to increase awareness and adoption. “Harnessing the power of our change agents created an effect where more people were engaged and thought of other creative applications for the use of interactive analytics. And then in turn, more people turn into change agents and bring more people and more ideas into the platform.”
eClinical Solutions also underwent a process for accelerating elluminate adoption with our internal Data Services team lead by Katrina Rice, Chief Delivery Officer of Data Services. In her Engage presentation, Katrina shared the eClinical story of elluminate Clinical Data Cloud adoption within our own services organization, including lessons learned with change management and the tangible results elluminate has demonstrated in speeding data reconciliation and accelerating LPLV (last patient last visit) to database lock cycle times.
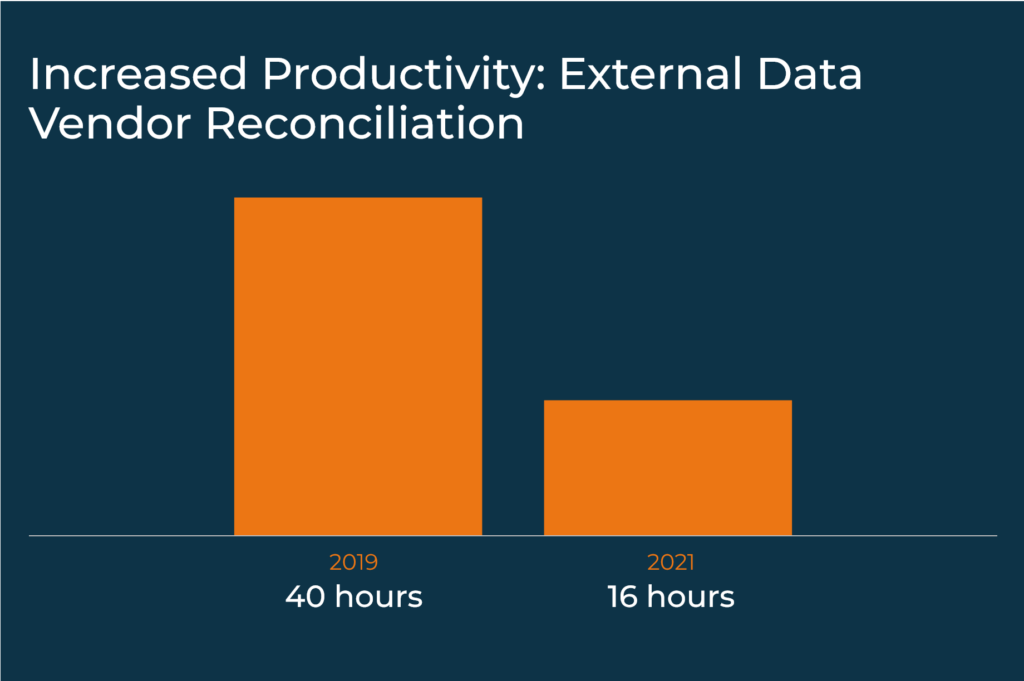
Katrina’s team sought to improve internal processes in order to deliver faster, more efficient productivity, quality, time, and service. Key steps taken to initiate adoption included building cross-functional diverse teams to assess processes, break them down and restructure as needed to incorporate new technologies. Internal communication was critical to ensure that on-going training was provided that demonstrated the value of elluminate and how specific roles can benefit from the product. Katrina explains that when rolling out a new technology, it’s important that you demonstrate not only why the product as a whole is great for the organization, but also how it will impact users at a singular level in their day-to-day work.
The results can be dramatic as Katrina presented with:
- 60% reduction in the time it takes to reconcile external data
- 50% decrease in LPLV to DBL to 2.5 weeks
elluminate Product Advancements: Risk-Based Quality Management (RBQM) and Clinical Trial Management System (CTMS Insights)
In addition to hearing from other elluminate users at Engage, eClinical Solutions also utilizes the conference to inform users of the elluminate product roadmap with new features coming to the platform and enhanced product capabilities. Sam Anwar, Chief Technology Officer, Robert Musterer, Vice President Product Management, and Nathan Johnson, Director of Data Engineering shared some of the exciting enhancements made to elluminate RBQM and CTMS Insights.
elluminate Risk-Based Quality Management
elluminate RBQM provides clinical teams with a single platform for all study data sources to support a more focused, data-driven monitoring effort that enables active oversight and improves the compliance of a clinical trial. Integrated with Data Central, elluminate RBQM proactively identifies potential issues the moment they arise, ensuring study data quality and integrity from start to finish. RBQM is the evolved version of the previous RBM module, which now supports site and country Key Risk Indicator tracking and analysis, as well as the ability to manage study Quality Tolerance Limits. Also added this year is the RACT (Risk Assessment and Categorization Tool) which allows users to manage a global library of risk assessment questions, maintain study-specific study risk tools, and review risk assessments.
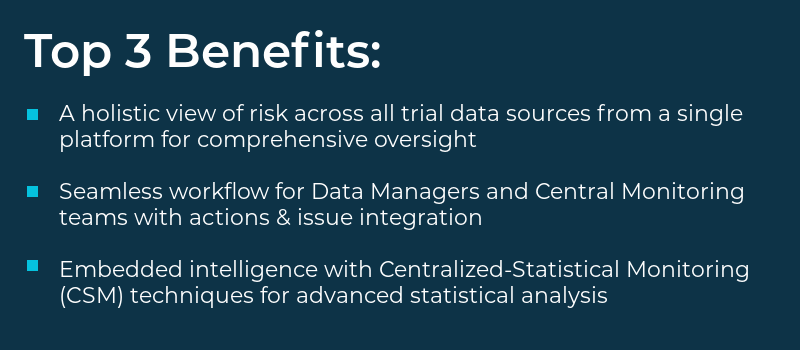
Top 3 Benefits
- A holistic view of risk across all trial data sources from a single platform for comprehensive oversight
- Seamless workflow for Data Managers and Central Monitoring teams with actions & issue integration
- Embedded intelligence with Centralized-Statistical Monitoring (CSM) techniques for advanced statistical analysis
Users can look forward to additional enhancements to RBQM, including building out the KRI library allowing you to bring in pre-defined risk indicators, and greater integration between RBQM and Data Central.
elluminate CTMS Insights
elluminate CTMS Insights helps enable clinical operations teams to spend more time proactively managing trials and risks across the portfolio instead of assembling stale data into trackers and status reports. Unlike other CTMS offerings which are primarily designed for in-sourced operational models, elluminate CTMS is designed for companies that outsource much of their operations to CROs and development partners.
Originally developed for Agios Pharmaceuticals and then launched broadly in early 2021, elluminate CTMS Insights functions as the data intelligence layer on top of the Operational Data Repository (ODR) that includes a wealth of operational data for clinical trials. During his Engage presentation, “Leveraging elluminate for Operational Data”, Nick Hargaden, Associate Director Clinical Systems at Agios, shared how his team improved efficiency and communication by utilizing a centralized data repository with elluminate and CTMS Insights. As Nick stated, “We’ve always felt like this IS our data, but it’s another thing to be able to bring it in-house so we can use it, combined with near-real time and accurate reporting that leverages ALL of our data, system and partner/vendor capabilities, but above all, following the Agios core principle to stay lean as we do this.”

Top 3 Benefits
- Reduced time to get data into the Operational Data Repository (ODR)
- Greater ease of deriving actionable insights
- Ease of conducting any enquiry of interest
Upcoming additions to CTMS Insights include more robust reporting through direct streams from the Clinical Data Repository (CDR) to the ODR, and forecasting capabilities allowing you to compare historical and planned data against actualities as well as predictive analytics.
elluminate as the Foundation for Digital Innovation and Decentralized Trial Models
Over the course of the elluminate Engage conference, we heard from 16 leaders at emerging to mid-size and large global life sciences organizations on why they trust elluminate with their most valuable clinical trial data. Rich Gleeson, Head of R&D Technology at Cerevel Therapeutics and Ted Snyder, Director of Clinical Informatics at Praxis Precision Medicines closed out the 2-day learning event with the panel, “Leveraging the elluminate platform to Solve Data Problems and Empower Clinical Teams in Emerging Biotechs”. When speaking on why Cerevel selected elluminate over other clinical data platforms, Rich Gleeson shared, “We wanted to be digitally transformed and we really needed a centralized single version of the truth. elluminate offered a cloud-based solution, rapid time to value with standard reports we got out of the box, and fertile ground to iterate over time based on our specific needs. Early on we needed a solution and elluminate fit that bill.”
elluminate can also serve as an essential tool when adapting to a decentralized trial model as the complexity and variety of data sources continues to grow. Ted Snyder shared that at Praxis, they are seeing more studies using upwards of 5 external vendors in addition to data being received from devices like wearables and sensors that provide a completely different scale of data. Ted explains, “Because our studies are getting more complicated and because we have more data streams coming in, we really need a platform like elluminate to bring all of those data sources together. I don’t know how you could run these trials without elluminate.” As clinical trials continue to push towards alternative models to reach more patients, elluminate can help bridge the data gap with robust reporting, visualizations, and trial oversight.
If you’d like to explore how elluminate can be implemented at your organization, we invite you to request a demo today.
Author

By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.







