
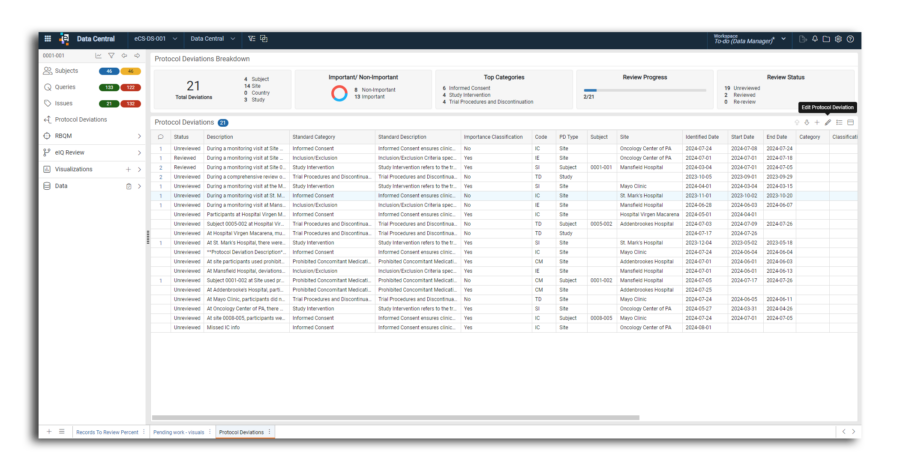
Protocol Deviations
Streamlining Management for Better Compliance and Insights
Managing protocol deviations is critical to ensuring trial integrity and participant safety. elluminate® Protocol Deviations centralizes all deviation data, streamlining ingestion, standardization, and trend identification across diverse sources, including CROs, CRAs, and CTMS systems. With AI-powered anomaly detection, Protocol Deviations automatically flags unusual patterns and potential compliance risks. By eliminating manual, siloed processes, sponsors can achieve real-time insights, enhanced oversight, and regulatory compliance.
Key Benefits of elluminate® Protocol Deviations
elluminate® Protocol Deviations delivers efficiency, quality, and compliance by automating data ingestion, centralizing deviation management, and providing actionable AI insights. Sponsors can make smarter decisions and ensure trial success with real-time analytics and standardized workflows.
Greater Efficiency
AI-powered ingestion and mapping minimize manual errors, while consolidating protocol deviation data from all sources into one platform streamlines management.
Enhanced Quality
Centralized data ensures consistent reporting and reduces discrepancies. Standardized Protocol Deviation Assessment Plans (PDAPs), powered by machine learning models that detect inconsistencies and data quality issues, enable uniform and accurate data review across studies.
Ensured Compliance
Real-time AI analytics support timely issue resolution and regulatory adherence, while standardized data and transparent workflows demonstrate sponsor oversight. AI-driven compliance monitoring proactively identifies potential regulatory risks.
Smarter Decisions
Interactive dashboards with AI-flagged deviations provide a comprehensive view of deviation metrics, including counts and trends, empowering data-driven decisions to mitigate future deviations.
Feature Highlights
elluminate® Protocol Deviations provides a comprehensive suite of tools, including standardized frameworks, customizable plans, and interactive dashboards, to ensure consistent, efficient, and insightful protocol deviation management across all studies.
Global PDAP: Establishes a standardized protocol deviation framework across all studies.
Study-Specific PDAP: Customizes deviation management for individual trials, inheriting global standards while allowing unique guidelines.
Interactive Dashboards: Deliver actionable AI insights into protocol deviations, ensuring efficient management and enhanced oversight.

Empowering Life Sciences Organizations for Trial Success
elluminate® Protocol Deviations equips sponsors and CRO organizations with the tools to address today’s data challenges. From ensuring data consistency to leveraging real-time insights and purpose-built AI, our platform empowers sponsors to deliver high-quality trials while meeting compliance standards efficiently.







