
Streamlining Digital Trials with a Modern Statistical Computing Environment (SCE)
In order to modernize their approaches, organizations have recently been turning to consortia like Transcelerate Biopharma, in addition to stalwarts like CDISC. Transcelerate’s Modernization of Statistical Analytics Framework aims to increase the use of modern statistical analysis technologies within the industry, enabling the faster delivery of innovative treatments to patients. Within this framework, three core principles for regulatory compliance are identified as follows:
1 Accuracy
The measure of correctness of software libraries that are used to generate results from a modern statistical analytics environment
2 Reproducibility
The ability to recreate statistical analysis outputs from the original dataset along with the associated environment, including all artifacts and dependencies
3 Traceability
The ability to trace inputs to outputs, providing evidence to connect e-data, code, and environment to the final output that is produced
Leveraging a Statistical Computing Environment with the key capabilities and functionality outlined below will ensure your organization is in line with digital approaches. Modern SCEs can make sure that these three principles of regulatory compliance will be met, while increasing development efficiency and streamlining program execution.
Program Development
- Full version control and access management
- Development lifecycle workflows
- Customizable folder structure within and accross studies
- Library of reusable code snippets, macros, and functions
- Intelligent editor with auto-complete and automation
Execution
- Integrated compute environments for SAS, R, and Python
- Support for package/ library validation
- Interactive real-time results or scheduled batch execution
- Log checking and display of results
- Full audit trail and reproducibility of results
Automation
- Dependency tracking for both data and inherited code
- Automated execution of programs based on data refresh
- Impact analysis and notification of data structural changes
- Auto-creation of programs based on configurable templates
- Data flow visualization from source output
Leveraging an Integrated SCE to Streamline Digital Trials
Demonstrating rigor in the analysis and reporting of clinical trial results requires transparency, reproducibility, and adequate documentation. With the increasing amount of clinical data available, more sophisticated methods, and an emergence of data science techniques, a high-performance computing environment is necessary in order to produce accurate and consistent results in support of regulatory submissions.

More companies are considering leveraging an e-clinical platform as they optimize and modernize their analytics and infrastructure strategy. Gartner defines an e-clinical platform as “an integrated suite of technologies connected to a platform architecture that provides services and solutions to manage clinical trial planning and execution”. 4 Utilizing a Statistical Computing Environment within an e-clinical platform that provides access to metadata, clinical data, and operational data can provide even greater benefits for programmers and statisticians. With access to these key elements, data, standards, and mappings can be leveraged for maximum reuse and increased programming and analysis efficiencies.
elluminate Statistical Computing Environment (SCE)
The elluminate SCE provides a validated environment for demonstrating rigor in statistical analysis and reporting. Unlike other cloud computing environments, elluminate SCE provides one centralized place for all your data, metadata, programs, and results — increasing the efficiency, traceability, and visibility of your statistical analyses. As a component of elluminate, the statistical computing environment enhances the value of the platform approach by leveraging data, standards, and mappings to maximize reuse and increase programming and analyses efficiencies.
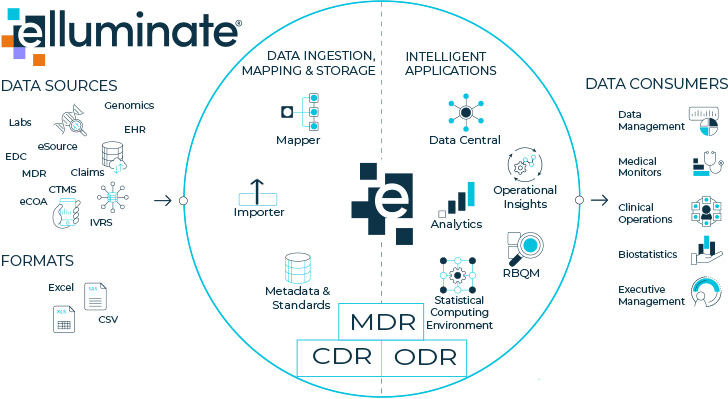
Fully integrated with the elluminate Clinical Data Cloud, elluminate SCE enables the production of submission or exploratory analysis outputs — within and across studies — in a way that is transparent, reproducible, efficient, auditable, automated, and secure.
Read The Digital Statistical Computing Environment (SCE) Playbook for Clinical Development to further explore how sponsors can leverage an integrated Statistical Computing Environment (SCE) to get in front of new clinical development challenges to streamline the production of submission deliverables while increasing programming and analysis efficiencies.








