
Four Key Elements of a Risk-Based Approach
There are several important elements of a risk-based quality management (RBQM) solution and depending on the complexity of the trial or analysis required, all or some of these elements can be incorporated into an RBQM strategy. Methods of implementing these components vary and are contingent to the technological infrastructure within an organization.
Risk Assessment and Categorization Tool (RACT): Risk assessment is a key component of risk management and is performed to identify potential risks impacting critical data and clinical trial processes. There are a few industry-specific risk assessment methodologies that can be utilized to perform a risk assessment such as the Transcelerate framework. With a Risk Assessment and Categorization Tool (RACT) that leverages a methodology similar to Transcelerate, it can be used to manage a library of risk assessment questions and maintain study-specific questions. A RACT can often be tailored to the protocol to ensure the appropriate questions are asked in order to best identify and assess study risk.
Key Risk Indicators (KRIs): Key Risk Indicators (KRIs) are metrics used to monitor identified risk throughout the duration of a trial, prioritizing patient safety and allowing for quicker detection and mitigation of potential issues. The number and type of KRIs selected depends on the protocol and complexity of the trial. KRIs are most effective and accurate when there is robust, real-time study data to feed these measurements.
Quality Tolerance Limits (QTLs): Quality Tolerance Limits (QTLs) are pre-determined limits for specific trial parameters that signal possible risk or further investigation when reached. Unlike KRI’s which are used to identify issues at the site performance level, QTLs are utilized to detect systemic deviations across a study. However, both KRIs and QTLs enable monitors to prioritize where to take immediate action.
Centralized Statistical Monitoring (CSM): With Centralized Statistical Monitoring (CSM), aggregated electronic clinical trial data can be reviewed remotely, eliminating the need for on-site monitoring. The value of CSM became even more apparent when on-site monitoring was not possible during the pandemic, leading to significant trial disruptions across the industry. With CSM, statistical algorithms can be applied to the aggregated trial data to enable the detection of anomalies and differentiate areas of concern with statistical significance.
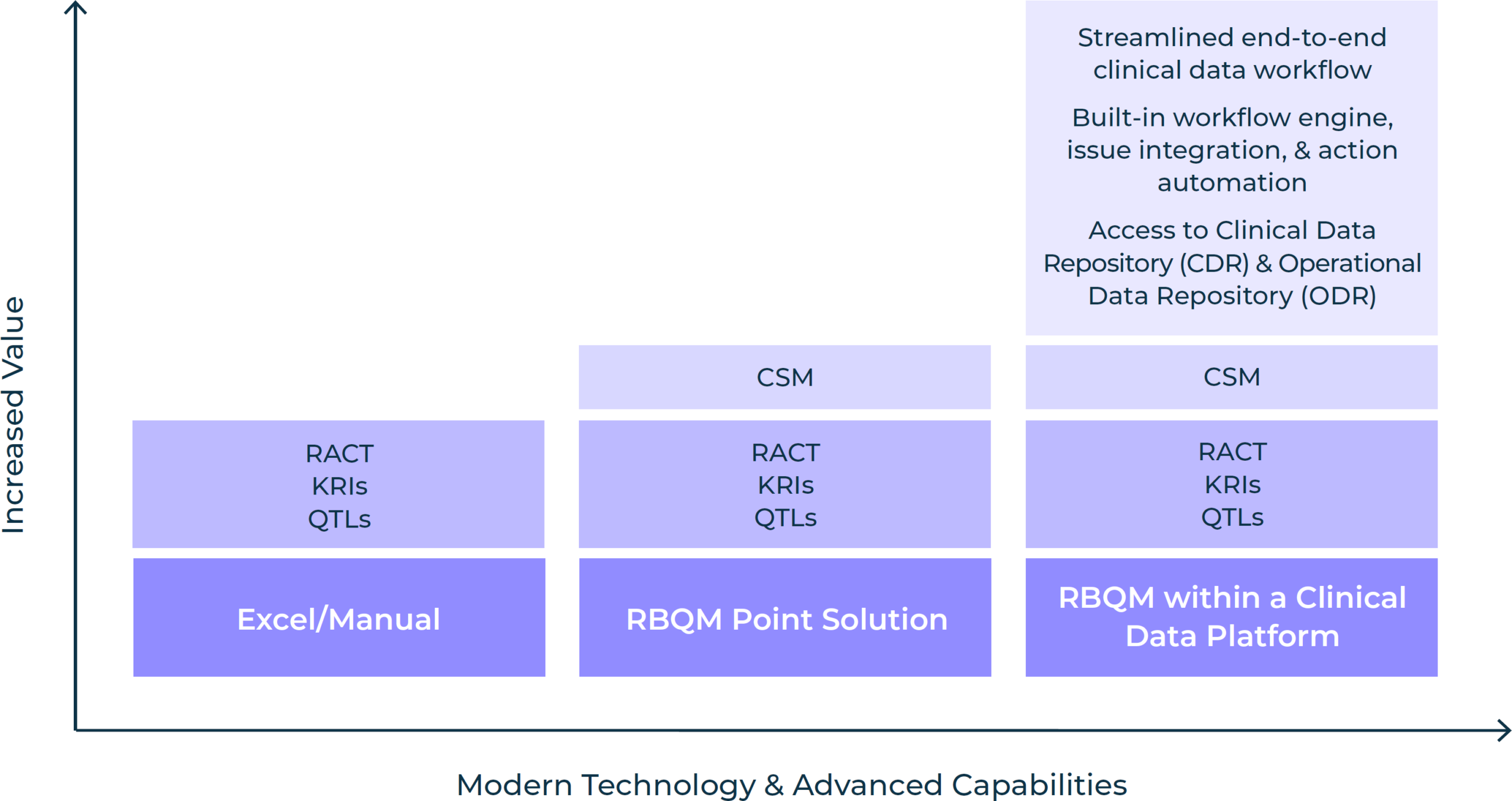
The methods by which some or all of these RBQM components are incorporated into a monitoring strategy has evolved over the years. Prior to the availability of RBQM point solutions, RACT, KRIs, and QTLs were primarily managed in spreadsheets or manual trackers. With point solutions, those components can be managed from a single system that also enables CSM. And while an RBQM point solution undoubtedly improves trial efficiency, taking a platform approach to RBQM provides access to all study data in real-time, enabling greater accuracy, advanced analytics, and better oversight. Read this white paper to learn how you can take a proactive approach to RBQM adoption for more efficient monitoring.

Establishing a Foundation for a Holistic RBQM Strategy
Access the White Paper
By submitting, you agree to the processing of your personal data by eClinical Solutions as described in our Privacy Policy.






